What is a sulfated battery and how do you prevent it?

Sulfation is the formation or build-up of lead sulfate crystals on the surface and in the pores of the active material of the batteries’ lead plates. If the battery is left unattended once sulfation starts, it will form larger crystals on the plates. Consequently, the battery capacity will get smaller and smaller as the sulfation builds. Eventually, it reaches a stage in which the battery may not work at all.
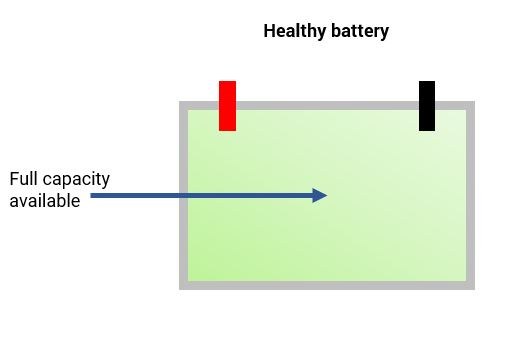
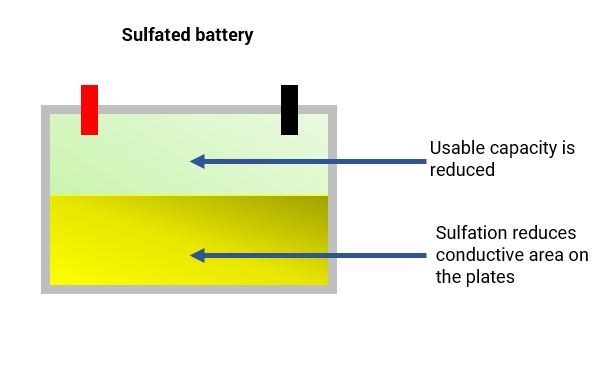
During normal use of the battery, the formation of lead sulfate crystals is only temporary. They disperse during the recharging process. The issue with sulfation occurs when the larger lead sulfate crystals become permanent.
TYPES OF BATTERY SULFATION
There are two types of sulfation in lead acid batteries, reversible (also referred to as soft) and permanent (also referred to as hard). Fortunately, their names are self-explanatory and imply the effects of sulfation to the battery. If you recognize sulfation early, an experienced professional can sometimes reverse it. However, a skilled technician should perform this process, as it involves overcharging the battery and can raise its temperature. For those interested, research on inverse charging for recovering sulfated lead-acid batteries is available here.
If you recognize sulfation early, an experienced professional can sometimes reverse it. However, this process involves overcharging the battery and should only be performed by a skilled technician.
If you catch sulfation early, a skilled professional can sometimes reverse it, though the process involves overcharging and should only be performed by an expert. Permanent sulfation builds-up when a battery has been in a low state-of-charge for weeks or months. At this stage, restoration of the battery or reversal of the sulfation, is highly unlikely. As a result, the battery will suffer the effects of permanent sulfation.
WHAT CAUSES A SULFATED BATTERY?
All lead acid batteries will suffer from sulfation during their lifetime as it is part of the chemical reaction of a battery. Sulfation builds up faster when the battery lacks a full charge, is undercharged, stored at high temperatures, or left unused for long periods. Additionally, storing the battery without fully charging it first accelerates sulfation.
Battery sulfation is the most common cause of early battery failure in lead acid batteries.
Applications which can suffer from battery sulfation more frequently than others include starter batteries for cars and powersport vehicles. This can be due to short or infrequent journeys, which do not give the battery sufficient time to charge. Off-grid renewable applications such as wind and solar can also suffer from sulfation. This is due to their intermittent nature, not guaranteeing a full charge to the battery.
HOW TO FIND OUT IF A BATTERY IS SULFATED
If sulfation affects your battery, it will lose efficiency. Common signs include poor or no charge retention, premature power loss, dim headlights, weak AC, or slow start-up. Externally, the battery may appear normal. To confirm sulfation, test the standing voltage with a multimeter: below 12.6 V for AGM batteries or 12.4 V for starter batteries indicates undercharging, often caused by sulfation.

THE EFFECTS OF A SULFATED BATTERY
Permanent battery sulfation can cause a variety of issues including:
- Loss of starting/cranking power
- Substantially longer charging times
- Increased heat build-up
- Shorter running times between charges
- dramatically shorter battery life
- complete battery failure
HOW TO PREVENT BATTERY SULFATION
The best way to prevent permanent battery sulfation is to maintain your lead acid battery, follow the recommended storage guidelines and follow lead acid battery charging best practices.
To prevent sulfation during storage, keep the battery charged to at least 12.4 volts and store it in an environment below 75°F (24°C). For every 10°F increase above this temperature, the battery’s self-discharge rate doubles.
Even if you are storing a new, fully charged lead acid battery you still need to ensure the voltage does not drop below 12.4 volts. Applying a top up charge (also referred to as a maintenance charge) periodically will help prevent sulfation building up.

Sulfation is the number one reason you should not store your lead acid battery without charge. Once sulfation of the lead plates has happened, reversing the effects is highly unlikely, so it is essential to look after your batteries from the start.



